According to a CNN analysis of Nuedexta prescription by doctors for nursing home residents, thousands of doctors (approximately half) received some sort of perk, either in the form of monetary payment or other compensation by the pharmaceutical company to promote Nuedexta use in nursing homes.
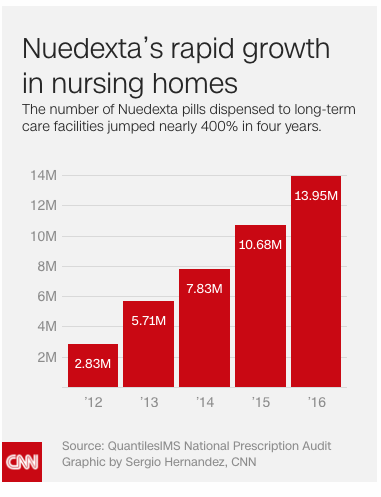
Nursing home residents are being prescribed a powerful drug that might not be necessary and could even be harmful. Made by Avanir (parent company Otsuka), Nuedexta use in nursing homes has increased by 400% in recent years. Nuedexta is a drug intended for an extremely rare condition called PBA, which impacts approximately five percent of the nursing home resident population.
Nursing home caretakers have determined that Nuedexta controls residents’ “mood disturbances” and reduces episodes of yelling out; consequently, some nursing homes and affiliated doctors have come up with ways to justify the drug’s prescription, even though the residents do not have PBA. In some cases, doctors are “diagnosing” residents with PBA, so that the prescription for Nuedexta will not be questioned.
Medicare Part D prescription drug funding is responsible for payments related to Nuedexta use in nursing homes; however, the funds are only disbursed if there is a diagnosis of PBA. According to the most recent data, Medicare spent over $137 billion on Nuedexta.
“Nearly half the Nuedexta claims filed with Medicare came from doctors who had
received payments or meals from the drugmaker.”
Presently, there are no FDA-approved, dementia-related agitation drugs. In the past, other drugmakers, such as a case against Abbott Laboratories in 2012, have been penalized for marketing drugs to control nursing home residents’ behavior, including but not limited to:
- Emotional and physical outbursts
- Restless behavior
- Dementia-and Alzheimers-related agitation
Dangers of Nuedexta Use in Nursing Homes
The Benefits of Increased Staffing
Many caretakers in the study believe that increased staffing in nursing homes would reduce the need for additional medication given to residents who suffer from these emotional/psychological behavioral issues. Understaffing is already a problem among nursing homes and has been shown to increase neglect, falls, and potentially death. Furthermore, Nuedexta does not come at a low cost. The drug’s price tag approaches $10,000 per year, increasing the already-strained financial burden most patients experience.
Contact Nursing Home Attorney Brian Murphy
If you believe your loved one has been unjustly placed on Nuedexta, is suffering from abuse and/or neglect in a Pennsylvania or New Jersey nursing home or assisted living facility, has developed unexplained bed sores, or is not being properly cared for, please contact Brian Murphy for a consultation. All matters are handled on a contingency fee basis.